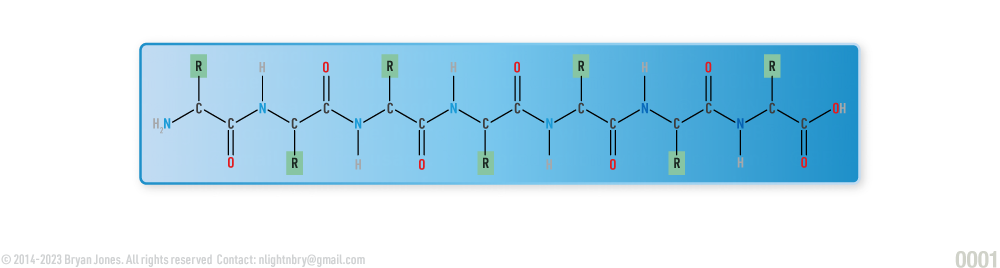
Protein
Proteins consist of groups of amino acids bonded together. Proteins change shape when stuff binds to them. Changing shapes can allow proteins to bind or unbind with other stuff.
Proteins are large molecules made up of long chains of building blocks called amino acids. There are 20 different types of amino acids that can be combined in different ways to make proteins with unique structures and functions.
Proteins are critical to the functioning of all living things. They play a role in nearly every cellular process, from metabolism and energy production to cell signaling and DNA replication. Some proteins act as enzymes, which catalyze chemical reactions in the body, while others act as structural components, forming important parts of cells and tissues.
The sequence of amino acids in a protein determines its three-dimensional shape, which in turn determines its function. Proteins can be composed of one or more polypeptide chains, and the way these chains fold and interact with each other can greatly affect the protein's function.
Proteins can also be modified after they are made, by adding or removing chemical groups. These modifications can affect the protein's stability, activity, and interactions with other molecules.
Overall, proteins are essential to life and are involved in a wide range of biological processes. They are studied extensively in the fields of biochemistry, molecular biology, and biotechnology.
Protein
Proteins consist of groups of amino acids bonded together. Proteins change shape when stuff binds to them. Changing shapes can allow proteins to bind or unbind with other stuff.
Proteins are large molecules made up of long chains of building blocks called amino acids. There are 20 different types of amino acids that can be combined in different ways to make proteins with unique structures and functions.
Proteins are critical to the functioning of all living things. They play a role in nearly every cellular process, from metabolism and energy production to cell signaling and DNA replication. Some proteins act as enzymes, which catalyze chemical reactions in the body, while others act as structural components, forming important parts of cells and tissues.
The sequence of amino acids in a protein determines its three-dimensional shape, which in turn determines its function. Proteins can be composed of one or more polypeptide chains, and the way these chains fold and interact with each other can greatly affect the protein's function.
Proteins can also be modified after they are made, by adding or removing chemical groups. These modifications can affect the protein's stability, activity, and interactions with other molecules.
Overall, proteins are essential to life and are involved in a wide range of biological processes. They are studied extensively in the fields of biochemistry, molecular biology, and biotechnology.
So, you know how your body needs food to grow and stay healthy, right? Well, one of the things your body needs from food is called "protein".
Protein is made up of tiny building blocks called "amino acids". Imagine if you had a bunch of Lego bricks and you could snap them together in different ways to make all kinds of cool structures - that's kind of like how amino acids come together to make protein.
When a bunch of amino acids snap together in a long chain, we call that chain a "polypeptide". So, you can think of a polypeptide as a long string of Lego bricks, all snapped together in a specific order.
Proteins do all kinds of important jobs in your body. They help your muscles move, they make up your hair and nails, and they even help your body fight off germs! So, the next time you have a tasty meal, remember that you're giving your body the protein it needs to stay strong and healthy.
Protein
Proteins consist of groups of amino acids bonded together. Proteins change shape when stuff binds to them. Changing shapes can allow proteins to bind or unbind with other stuff.
Proteins are large molecules made up of long chains of building blocks called amino acids. There are 20 different types of amino acids that can be combined in different ways to make proteins with unique structures and functions.
Proteins are critical to the functioning of all living things. They play a role in nearly every cellular process, from metabolism and energy production to cell signaling and DNA replication. Some proteins act as enzymes, which catalyze chemical reactions in the body, while others act as structural components, forming important parts of cells and tissues.
The sequence of amino acids in a protein determines its three-dimensional shape, which in turn determines its function. Proteins can be composed of one or more polypeptide chains, and the way these chains fold and interact with each other can greatly affect the protein's function.
Proteins can also be modified after they are made, by adding or removing chemical groups. These modifications can affect the protein's stability, activity, and interactions with other molecules.
Overall, proteins are essential to life and are involved in a wide range of biological processes. They are studied extensively in the fields of biochemistry, molecular biology, and biotechnology.
So, you know how your body needs food to grow and stay healthy, right? Well, one of the things your body needs from food is called "protein".
Protein is made up of tiny building blocks called "amino acids". Imagine if you had a bunch of Lego bricks and you could snap them together in different ways to make all kinds of cool structures - that's kind of like how amino acids come together to make protein.
When a bunch of amino acids snap together in a long chain, we call that chain a "polypeptide". So, you can think of a polypeptide as a long string of Lego bricks, all snapped together in a specific order.
Proteins do all kinds of important jobs in your body. They help your muscles move, they make up your hair and nails, and they even help your body fight off germs! So, the next time you have a tasty meal, remember that you're giving your body the protein it needs to stay strong and healthy.
Protein Basics
The primary structure of a protein is simply the linear arrangement, or sequence, of the amino acids that compose it.
Proteins are made up of 3 or more amino acids strung together. When three or more amino acids are connected, they are known as a polypeptide.
When a 3 or more amino acids snap together in a long chain, we call that chain a "polypeptide". So, you can think of a polypeptide as a long string of Lego bricks, all snapped together in a specific order.
Polypeptides are like necklaces made out of amino acids! They can be very short or very long, depending on how many amino acids are in the chain.
Polypeptide

Transcription and Translation

Amino Acids
Proteins are built from sets of amino acids. Each amino acid has a unique side group. A side group is also known as an R group, which will be explained shortly.
While some amino acids have side groups which are charged positively or negatively. The largest groups of amino acids are nonpolar. The molecular makeup of amino acid side groups is necessary to understand protein structure because these side groups bond with each other to give individual proteins specific shapes.
For now think of Amino acids as a polyamorous relationship
Generalized Amino Acid Structure:

Amino acids can have nonpolar covalent side groups.
Amino acids with nonpolar-covalent side groups will not react with water, because water is polar. Instead, the nonpolar side groups will cause amino acids to clump together in water, just as oil in water.
Amino acids can have polar covalent side groups.
Amino acids with polar-covalent side groups behave like water: one side of the molecules carries a weak (½) positive change (hydrogen) while the other side has a weak (½) negative charge (oxygen). These types of amino acid side groups are described as “non-ionic” because their electric charges are only a fraction of the full charge found on an ion.
Amino acids can have ionic side groups.
Amino acids with ionic side groups are usually fully charged (ionic), although the acidity of the surrounding solution can change this. Because of this, they are strongly attracted to the ionic side groups of other amino acids. They are also strongly attracted to water, which is polar and orients toward the opposite charge of the amino acid.
20 amino acids in Zwitterion form

20 amino acids found in nature
Aspartic
(Asp)
acidic

Asparagine
(Asn)
neutral, polar

Arginine
(Arg)
basic

Aspartic
(Asp)
acidic

Threonine
(Thr)
neutral, polar

Valine
(Val)
neutral, nonpolar

Proline
(Pro)
neutral, nonpolar

Phenylalanine
(Phe)
neutral, nonpolar

Histidine
(His)
basic

Lysine
(Lys)
basic

Glycine
(Gly)
neutral, nonpolar

Glutamic
(Glu)
acidic

Cysteine
(Cys)
neutral, polar

Tryptophan
(Trp)
neutral, nonpolar

Tyrosine
(Trp)
neutral, nonpolar

Serine
(Ser)
neutral, polar

Amino Acid Structure
Amino acids in proteins are comprised of the following:
The amino acids in proteins contain at least one carboxyl (COOH) functional group and one amine (NH2) functional group both attached to the same carbon atom. That carbon atom is called an Alpha-carbon (Cα). A carboxyl group behaves as an acid by contributing an H+ ion to a solution. An amine group behaves as a base by picking up an H+ ion from a solution; they carry a positive charge.
Generalized Zwitterion Amino Acid Structure:

Amino Group
Amino groups act as acids and carry a positive charge. Amino groups accept hydrogen ions (H+) in a solution.
It's worth noting that the term "amino" can be used as a prefix to describe other compounds or functional groups that contain an amino group (-NH2). For example, "amino sugars" are carbohydrates that contain an amino group in place of a hydroxyl group.
Amine refers to a class of organic compounds derived from ammonia (NH3) by replacing one or more hydrogen atoms with alkyl or aryl groups. Amines are characterized by the presence of a nitrogen atom bonded to one or more carbon atoms. They can be classified as primary, secondary, or tertiary amines based on the number of alkyl or aryl groups attached to the nitrogen atom
When you combine the amino functional group with another you get Amine group, but when it's by itself people say Amine. For now you get an Amino group and since it's bonded to you, you have to interact with it.
When you combine this group with another you get Amino, but when it's by itself people say Amine. Not to be confused with † which is used as a footnote marker, that may come later, for now you get an Amino group and since it's bonded to you, you have have to interact with it.
Amine group Structure:

Carboxyl Group
A carboxyl group acts as an acid and carries a negative charge.
A Carboxyl group belongs to the carboxylic chemical class. The group acts as an acid and carries a negative charge (this group pulls on anything with a positive charge).
Carboxylic acids are typically weak acids, meaning that they only partially dissociate into H+ cations and RCOO− anions in neutral (pH 7.0) aqueous solution. Carboxyl groups readily accept hydroxide ions (OH-).
A carboxyl group consists of a carbonyl group (a carbon double-bonded to an oxygen) and a hydroxyl group (an oxygen single-bonded to a hydrogen) attached to the same carbon atom. The general chemical formula for a carboxyl group is -COOH.
A Carboxyl group is like lips double-bonded to a carbon atom while stuck to a hydroxyl group (-OH), which is really a HO but they tell everyone they're a hydroxide Ion (not attached). Without the HO, it's just a carboxyl group which is oxygen sucked tight to a carbon atom.
Carboxyl Group Structure:

Side Group (R)
a Side group, also known as R group, is the distinguishing feature of an amino acid. The "R" stands for "remainder"."
The shape of the R group determines the specific properties of amino acids in proteins. An R group can be a hydrogen atom, an unbranched or branched chain of atoms, a cyclic ring structure (all carbon) or a heterocyclic structure (which includes other atoms in addition to carbons).
Amino acids are like a polyamorous relationship, there's a lot going on and always looking for an interesting experience. Well the group decided to dress as pirates one night, and began actively saying R, Rrr, Rrrr. With a mouth full of an R group which can really be anything, a bit Risqué. I guess they're into aspartic acid. The R stands for "Remainder" group, and not a group of pirates.
A table off to the side full of possibility just waiting for connection, connection depends on the group. Amine & carboxyl slides into place nicely as long as you don't pick up Tryptophan.
Generalized Amino Acid Structure:

Hey come over here R, let's mingle. What are you interested in? Asparagine sometimes Glu (Glutamic Acid). Amino says that's nice, I was thinking you're more aromatic, I can accept that. Carboxyl says you Tryptophan and hisses away with Histidine.
Example of where a side group connects:

Zwitterion
Zwitterions are neutral molecules with positive and negative charges - one on each end. Dipolar ions are the same as zwitterions. Depending upon its environment, a zwitterion can become acidic or basic.
Solutions are serious, so pay attention to what you drink, your body operates within a curve (statistical curve, not to be confused with testicular), and being on the edges of that curve is not fun. You want to be in the middle of that curve, moving around and enjoying life. Although when you've consumed a lot of alcohol you may find yourself on the edge of that curve surrounded by HOs (hydroxide ions)
Zwitterion Structure:

Peptide Bond Formation
Peptide bonds are the bonds between the amino acids.
In order to link amino acids together a dehydration synthesis has to occur. The carboxyl group of one amino acid must be positioned next to the amine group of another. A water molecule is then removed as the carboxyl-group carbon atoms bond to the amino-group nitrogen of its neighbor. The resulting covalent linkage is called a peptide bond.
Dehydration synthesis of amino acids:

A peptide bond is a covalent bond that forms between the carboxyl group (-COOH) of one amino acid and the amino group (NH2) of another amino acid during the condensation or dehydration synthesis reaction that links amino acids together to form a polypeptide chain. The resulting peptide bond has a rigid planar structure due to the partial double-bond character resulting from resonance.
Dehydration synthesis of amino acids:

Hydration synthesis of amino acids:

Resonance in Peptide Bonds
The stability of the peptide bond is due to the resonance of amides.The term "peptide resonance bond" refers to the characteristic nature of the peptide bond, which exhibits resonance or delocalization of electrons across the peptide bond.
In a peptide bond, the carbon atom of the carbonyl group (C=O) and the nitrogen atom of the amino group (N-H) share a pair of electrons to form a covalent bond. However, the electrons involved in the peptide bond are not localized solely between the carbon and nitrogen atoms, but they are also delocalized or shared among adjacent atoms along the peptide backbone.
Resonance in a peptide bond:

Because the bond between the carbonyl carbon and the nitrogen has a partial double bond character, rotation around this bond is restricted. Thus, the peptide unit is a planar, rigid structure.
(+) Did you install a rotating bed?
(–) Yes, but it only rotates a little bit. This bond is like ball-bearings, extra smooth and lubricated and ready to fold like someone whose in too deep. Do you feel that?
(+) I feel like I'm vibrating.
(–) Yeah that's the delocalized electrons.
(+) I thought It might have been your "faded bond line", you know they make rechargeable ones?
(–) Yes.
Amino Acid Residue
A protein residue is the remaining part of an amino acid after forming a peptide bond. It consists of the amino group, carboxyl group, and side chain. The side chain, which varies among amino acids, gives proteins their unique properties.
The term "residue" typically refers to the remnants or remains of proteins that are left behind after a chemical reaction or biological process. It can also refer to the specific amino acid residues that make up a protein.
For example, the amino acid residue alanine has a side chain that is simply a hydrogen atom. The amino acid residue arginine has a side chain that is a positively charged guanidinium group. These two amino acid residues have very different properties, and they would be found in different types of proteins.
The amino acid residues in a protein are linked together by peptide bonds. Peptide bonds are formed when the carboxyl group of one amino acid reacts with the amino group of another amino acid. This reaction releases a molecule of water.
The sequence of amino acid residues in a protein is called the primary structure of the protein. The primary structure of a protein determines its shape and function. The different types of protein residues are classified according to the properties of their side chains.
Four main classes of protein residues:
- Hydrophobic residues have side chains that are nonpolar and tend to avoid water. These residues are often found in the interior of proteins, where they are protected from water.
- Hydrophilic residues have side chains that are polar and tend to interact with water. These residues are often found on the surface of proteins, where they can interact with other molecules in the cell.
- Charged residues have side chains that have a positive or negative charge. These residues can interact with other charged residues in the protein or with charged molecules in the cell.
- Specialty residues have side chains that have special properties. For example, the side chain of the amino acid cysteine can form a disulfide bond with the side chain of another cysteine residue. Disulfide bonds help to stabilize the structure of proteins. The different types of protein residues interact with each other in different ways. These interactions help to determine the shape and function of proteins.
Bonds Within a Protein
The 3-dimensional structure of a protein's polypeptide chain or chains may be locked in place by other stronger bonds. These bonds are formed between components of the side group (-R residues) of the amino acid.
You’ve just straightened your hair and it feels great. Next stop, the bar where you can show it off. After an hour of socializing your hair has gone flat well that’s because your hair is made of proteins, and bonds are starting to occur now that you hair has hit a cloud of atmospheric gas and (H2O). Think of it this way, you got some balls stuck to your hair now and from your perspective that’s not where you want them.
Types of bonds within proteins include:
- Ionic bonds or salt bridges - between negatively charged and positively charged side groups
- Hydrogen bonds - between -COOH and-NH2 and -OH groups on side chains
- Hydrophobic forces - between non polar side groups
- Disulfide bonds - between thiol groups on pairs of the amino acid cysteine
Hydrogen Bond:

Ionic Bond:

Disulfide Bond:

Van Der Waals Attraction:

Protein Structure
Proteins come in different shapes and sizes: Primary, Secondary, Tertiary, and Quaternary.
Oh look at the pretty neck choker, that's a really nice blue color, hopefully your face doesn't turn blue because I want to see what other shades you come in. I suppose you need something reddish on the other end, and that's called a carboxyl group, and when you combine that with your blue amino group you get a ribbon.
Primary Structure: polypeptide strand

Secondary Structure: helix | These are Ribbon Lacing stockings, of which he licks so it's a Helix stocking. Don't confuse the "hairy" single-bond lines with your shaving routine, because in reality those bonds lines aren't there. But for real, keep them smooth this way I'll feel like I'm eating desert and not a desert. You know I'll notice, then you may be all self-conscious and dry-up.
Secondary Structure: helix

Secondary Structure: pleated sheet | If the last was Ribbon Lacing stockings, then these are pleated skirts you wear for fun. Cheerleaders are covered in protein one way or another, so support your local sport teams and think about proteins, buy a hot dog while you're at it.
Secondary Structure: pleated sheet

Tertiary Structure: folded helix and pleated sheets | Here we have the Ribbon Lacing Helix stockings and the pleated skirt combined. Sometimes you have multiple helix and pleated proteins in one structure just like cheerleaders. So now you have a team of Cheerleaders covered in protein moving around, interacting with one another to do some really fascinating stuff like bending, twisting, or simply bonding with each other. As you might imagine, sometimes a pleated protein can make or break bonds, but that's okay others will form very quickly. Just wait until you realize what cell adhesion looks like, you may begin to regret some life choices, but I can tell you the more protein you consume the better your face will look.
Tertiary Structure: folded helix and pleated sheets

Structural and functional domains are modules of tertiary structure. The tertiary structure of proteins larger than 15,000 monomers is typically subdivided into distinct regions called domains. These domains are compactly folded polypeptide region. These regions are physically separated from one another but can be connected by intervening segments of the polypeptide chain.
Tertiary Structure: Conventional representation of folded helix and pleated sheet

Your fingers are running through your hair and it feels silky smooth. You hair cuticle comprised of keratin, rich with mild acidic cysteine groups. When your hair is washed, your hair releases protons (deprotonation), giving hair a negative charge (-). Squirt some conditioner out on your hand and glide that hand back and forth allow those quaternary ammonium compounds and polymers to attach bond to your hair due to electrostatic interactions, once attached they produce several effects. Hydrocarbon backbones help lubricate the hair surface giving it additional structure, reducing the sensation of roughness and facilitating easier combing. Positively changed groups coat your hair preventing individual hairs from clumping together because each hair is positively charged and repel each other.
Are bonds the same as electrostatic interactions?
No, bonds and electrostatic interactions are not the same. Bonds are a type of electrostatic interaction, but not all electrostatic interactions are bonds.
Bonds are a type of chemical bonding that results from the sharing of electrons between atoms. There are two main types of bonds: covalent bonds and ionic bonds.
Electrostatic interactions are any type of interaction between charged particles. There are many different types of electrostatic interactions, including:Ionic bonds, Hydrogen bonds, Dipole-dipole interactions, London dispersion forces
I suppose a Cheerleading competition is one too many times to use this analogy, butt it works. I suppose after all that bending and twisting you're now hungry, so buy a pretzel and if you did forgot to shave your legs, maybe ask for it without butter. I'm going to get some lipids for this.
Quaternary Structure: two or more tertiary structures in their folded states

Protein Denaturation
Denaturation is a process by which some external compound or stress causes a protein to lose structural integrity.
The structures affected by denaturation include the quaternary structure, tertiary structure and secondary structure, all of which exist in the native state of the protein. Denaturation of proteins in a living cell can result in the death of a cell or a disruption of a cell’s behaviors.
Did your straightened hair go from fluff to flat, well when you used all that heat to straighten your proteins you just denatured them.
Denatured proteins can:
- Experience conformational change (a change in the shape of a macromolecule)
- Lose solubility
- Aggregate (come together of misfolded proteins) from exposure to hydrophobic groups
Denaturation can be caused by the following:
- Strong acid or base
- Concentrated inorganic salt
- Organic solvent
- Radiation or heat
Salts and heavy metal ions, such as mercury, silver, and lead These ions form strong bonds with the carboxyl groups (COOH) of acidic amino acids or sulfhydryl groups (SH) of cysteine, disrupting ionic bonds and disulfide bonds.
Organic Solvents These compounds can disrupt hydrogen bonding within a protein.
Have you ever bleached your hair using hydrogen peroxide or ammonia? Well this breaks bonds and can cause long-term damage if down improperly, the result is thin, less curly hair in which won't renature, your hair is also prone to breakage because you weakened the structure of your hair cuticle.
Bleaching your hair breaks the chemical bonds in the hair's cortex, which is the middle layer of the hair shaft. The cortex contains melanin, which gives hair its color, and keratin proteins, which give hair its strength and structure.
The bleaching process involves using hydrogen peroxide and ammonia or other alkaline substances to lift the hair cuticle and penetrate the cortex. The hydrogen peroxide reacts with melanin and breaks down its structure, causing the color to lighten. This process also breaks down the keratin proteins, which weakens the hair's structure and makes it more prone to breakage.
The bleaching process can also cause damage to the hair's outer layer, the cuticle, which can lead to further damage and breakage. It is important to take proper care of bleached hair to minimize damage and breakage, such as using protein treatments, deep conditioning, and avoiding excessive heat styling.
Heat (about 50 degrees C) or ultraviolet radiation Heat and UV radiation are two types of kinetic energy which cause the atoms in protein molecules to vibrate more vigorously. This vibration disrupts Van der Waals forces (either relatively weak hydrogen bonds or the opposite, dispersion forces).
Protein:

Protein Functions
Proteins can be broadly categorized into several types based on their structure, function, and location in the body. Some of the main categories of proteins include:
| Category | Function |
|---|---|
| Enzymatic | These are proteins that catalyze chemical reactions in the body. Enzymes are essential for metabolism, digestion, and other biochemical processes. |
| Structural | These proteins provide support and shape to cells and tissues. Examples include collagen, elastin, and keratin. |
| Storage | Storage of amino acids. |
| Transport | These proteins move molecules, such as oxygen and nutrients, throughout the body. Examples include hemoglobin and transferrin. |
| Hormonal | Coordination of an organism’s activities. These proteins act as chemical messengers in the body, regulating various physiological processes. Examples include insulin and growth hormone. |
| Receptor | Response of cell to chemical stimuli. |
| Motor | These proteins are responsible for movement and contraction in cells and tissues. Examples include myosin and actin. |
| Antibodies | Protection against disease. These proteins help the immune system recognize and fight off foreign invaders, such as viruses and bacteria. |
| Regulatory | These proteins help to control gene expression and other cellular processes. Examples include transcription factors and protein kinases. |
*These categories of proteins are not mutually exclusive, as many proteins can have multiple functions and roles in the body. Additionally, there are many other subcategories of proteins that have specific functions and properties.
Conjugated Proteins
| Class | Prosthetic Group | Example |
|---|---|---|
| Flavoproteins | Flavin nucleotides | Succinate dehydrogenase |
| Glycoproteins | Carbohydrates | Immunoglobulin G |
| Hemoproteins | Heme (iron porphyrin) | Hemoglobin |
| Lipoprotein | Lipids | ϐ1-Lipoprotein of blood |
| Phosphoproteins | Phosphate groups | Casein of milk |
| Metalloproteins | Calcium | Calmodulin |
| Copper | Plastocyanin | |
| Iron | Ferritin | |
| Zinc | Alcohol dehydrogenase |
May help you remember 6 classes of conjugated protein.Car bo drinkin water, hi and glittered. Who dis glob? He me on your lip, rippled flavor may need faucet for pho soup unless you like metal in your microwave.
Assembly of a Protein
Assembling a functioning protein involves several steps, including:
- Transcription: The DNA sequence is transcribed into messenger RNA (mRNA) by RNA polymerase.
- Translation: The mRNA sequence is translated into a protein sequence by ribosomes.
- Folding: The protein chain folds into its correct three-dimensional shape through a process called protein folding.
- Post-translational modifications: The protein may undergo various modifications, including phosphorylation, glycosylation, and cleavage, which can affect its function and stability.
- Assembly: Multiple protein chains may assemble together to form a functional protein complex, which can perform specific biological functions.
- Transport: The protein is transported to its proper location within the cell or secreted outside the cell.
- Binding to ligands: The protein may bind to various ligands, such as other proteins or small molecules, to perform its specific function.
- Regulation: The activity of the protein may be regulated through various mechanisms, such as feedback inhibition, phosphorylation, or gene expression.
Chaperones help fold proteins correctly
Proteins that ensure correct polypeptide folding are called molecular chaperones or simply chaperones. They assist in the proper folding of newly synthesized proteins and help to prevent the formation of misfolded or aggregated proteins, which can lead to diseases such as Alzheimer's, Parkinson's, and Huntington's. Chaperones also play a role in the assembly and disassembly of protein complexes and in the transport of proteins across membranes. Some well-known chaperones include heat shock proteins (HSPs), chaperonins, and prefoldin.
Covalent modification of proteins
The process by which certain functional groups, such as phosphate, acetyl, methyl, or ubiquitin groups, are covalently attached to specific amino acid residues on a protein. This modification can alter the activity, localization, stability, or interaction of the protein. Some common covalent modifications include phosphorylation, glycosylation, acetylation, methylation, and ubiquitination.
- Phosphorylation, the addition of a phosphate group to a serine, threonine, or tyrosine residue, is one of the most common types of covalent modification. It can activate or inactivate a protein, or alter its interactions with other proteins or molecules.
- Glycosylation, the addition of a sugar molecule to a serine, threonine, or asparagine residue, can modify the activity, folding, or stability of a protein, or determine its localization or interaction with other molecules.
- Acetylation, the addition of an acetyl group to a lysine residue, can alter the protein's activity, stability, or interaction with other proteins or molecules.
- Methylation, the addition of a methyl group to a lysine or arginine residue, can affect the protein's activity, localization, or interaction with other proteins or molecules.
- Ubiquitination, the addition of a ubiquitin molecule to a lysine residue, can target the protein for degradation or alter its activity, stability, or localization.
Covalent modifications of proteins are reversible and dynamic, and play important roles in regulating cellular processes such as signal transduction, gene expression, protein degradation, and cell cycle progression.
Viral Proteins
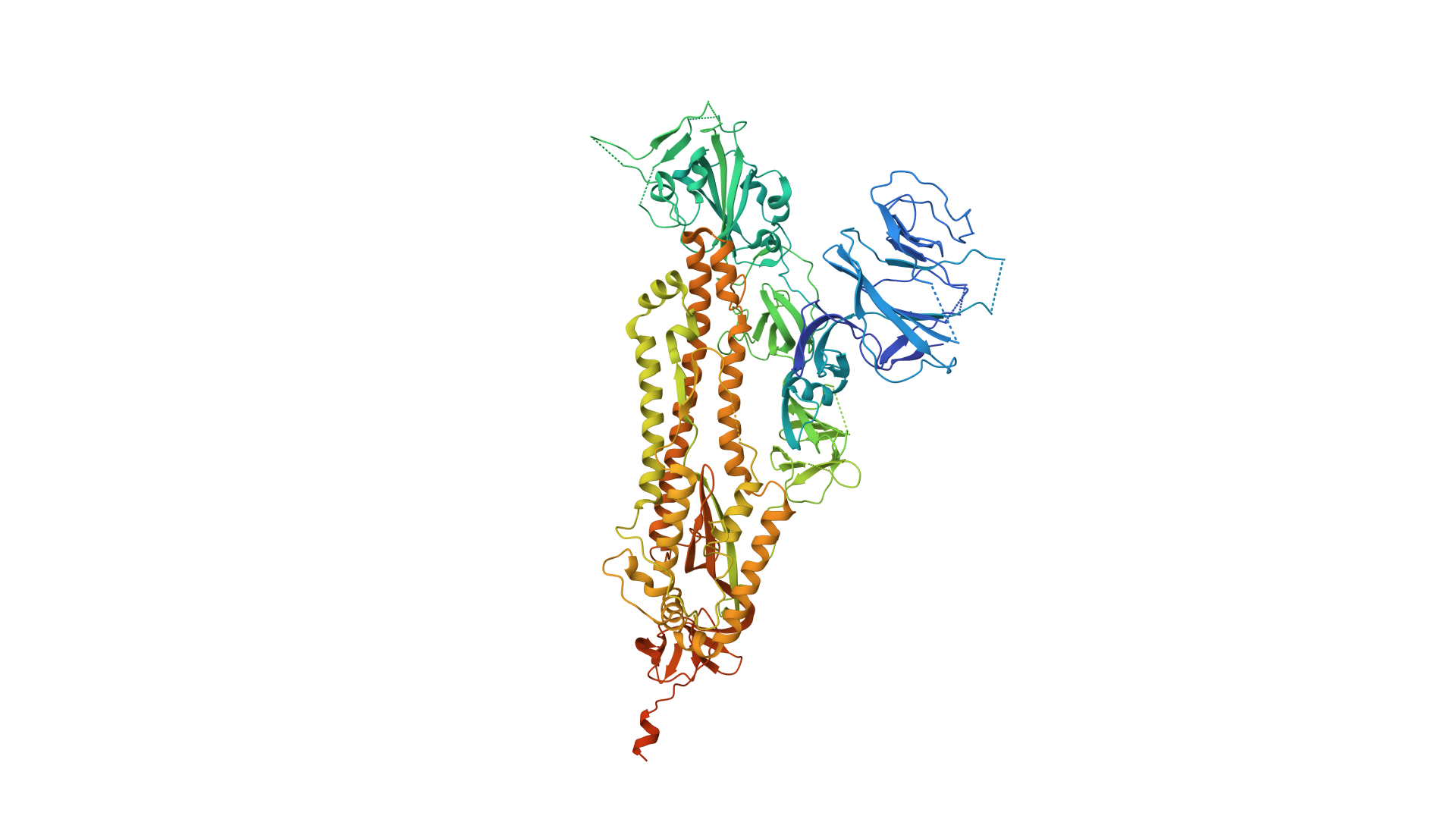
The 2019 Coronavirus (2019-nCoV) is an example of how viruses (viri) also contain proteins which are used to gain access to a cell.
2019-COVID spike glycoprotein with a single receptor-binding domain - Side view
2019-COVID spike glycoprotein with a single receptor-binding domain - Top view
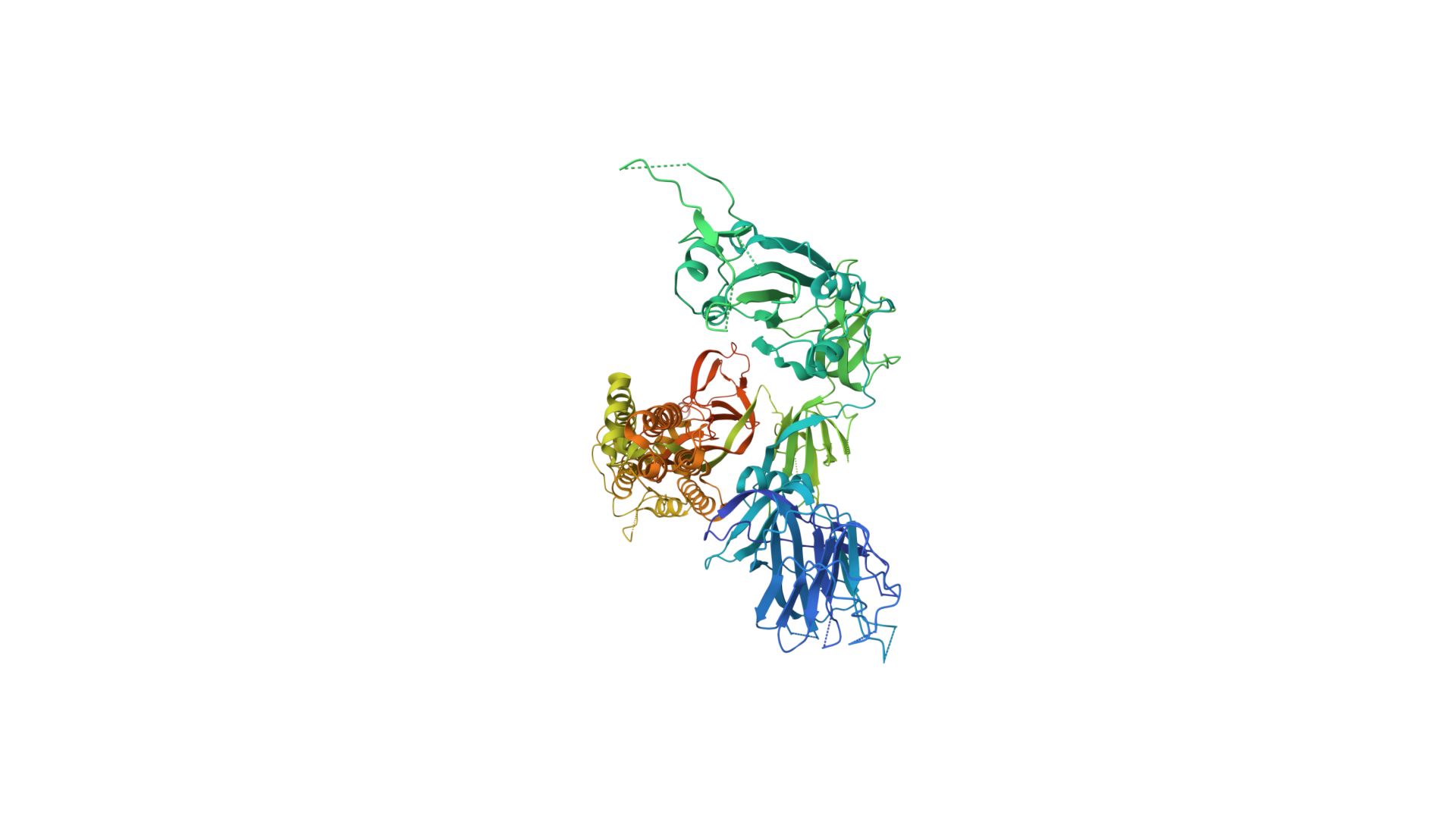
The following graphic represents the secondary structures of a COVID spike protein. This "spike" allows a virus to dock with receptors located on a cell's membrane, which is why proteins such as these are mapped. By mapping the viral amino acid sequence scientists and engineers can produce vaccines that target these specific areas of a protein and neutralize it.
There are three regions within this sequence that allow the viral protein to bond, while the remaining sequence provides structure and other attributes such as hydropathy.
The grey letters in a protein sequence indicate uncertainty about the identity of the amino acid at that position. This uncertainty can be due to a number of factors, such as incomplete data or ambiguity in the experimental methods used to determine the structure of the protein. Grey letters are typically used to represent amino acids that have been assigned a confidence score of less than 50%. This means that there is a greater than 50% chance that the amino acid at that position is not correct.
COVID Spike Glycoprotein

The following is the same protein but instead of 1 chain (sequence of aminoacids), it's all 3 chains combined which form a polymer consisting of multiple chains.
The Grouch Protein
Here we have a “trashcan” (sheet proteins) filled with H2O acting as a "pool" with the “green guy” (CRO) inside. Together they make a Fluorescent Protein that glows in the dark named, Green Fluorescent Protein (GFP) From Aequorea Victoria.
The fluorophore in GFP can be in two states: neutral and ionized. The neutral state absorbs at 395 nm, while the ionized state absorbs at 475 nm. The equilibrium between these states is governed by a hydrogen bond network. In the S65T structure, the extra methyl group sterically hinders the hydrogen bond network, which prevents the ionization of Glu-222. Therefore, the fluorophore is permanently ionized, resulting in only a 489 nm excitation peak.
The peptide derived chromopore CRO is an assembly of three amino acids, Tyrosine, Glycine, and Threonine. It's structure essentailly makes this protein fluoresce. The CRO structure is stablized by additional amino acids, H2O and electrostatic forces that enable continuous 489nm emission.
CRO | 1EMB

Dual Absorption Wavelength of Protein | 1EMB

The crystal structure of wild-type green fluorescent protein (GFP) at 2.1 Å resolution, together with the structure of the Ser-65-to-Thr (S65T) mutant, explains the dual wavelength absorption and photoisomerization properties of GFP. The two absorption maxima are caused by a change in the ionization state of the chromophore. The equilibrium between these states is governed by a hydrogen bond network that allows protons to be transferred between the chromophore and neighboring side chains.
The neutral form of the fluorophore, which absorbs maximally at 395 nm, is stabilized by the carboxylate group of Glu-222 through electrostatic repulsion and hydrogen bonding with a bound water molecule and Ser-205. The ionized form of the fluorophore, which absorbs at 475 nm, is present in a minor fraction of the native protein. Glu-222 donates its charge to the fluorophore by abstracting a proton through a hydrogen bond network that also involves Ser-205 and bound water. The ionized state of the fluorophore is further stabilized by a rearrangement of the side chains of Thr-203 and His-148.
UV irradiation causes a shift in the ratio of the two absorption maxima by pumping a proton from the neutral chromophore's excited state to Glu-222. The loss of the Ser-205-Glu-222 hydrogen bond and the isomerization of neutral Glu-222 explain the slow return to the equilibrium dark-adapted state of the chromophore.
In the S65T structure, steric hindrance by the extra methyl group stabilizes a hydrogen bonding network that prevents the ionization of Glu-222. Therefore, the fluorophore is permanently ionized, resulting in a 489 nm excitation peak.
CRO | 1EMB

CRO interacting with Hydrogen Donor / Hydrogen Acceptor | 1EMB

Orient your view toward the yellow Sulfur sphere, below is a red sphere which is an Oxygen atom surrounded with protonated Hydrogen atoms which aren't visible. Dashed-lines indicate interactions with CRO which is to the right of the yellow Sulfur atom.
In summary, the center molecule (CRO) is similar to an alternative current (AC) due to water, hydrogen bonding and electrostatic forces.
Zinc Fingers
Zinc fingers are small protein domains that bind DNA and regulate gene expression.
Here is a more detailed summary:
- Zinc fingers are small protein structural motifs that are characterized by the coordination of one or more zinc ions.
- They are found in many different proteins, including transcription factors, RNA binding proteins, and DNA repair proteins.
- Zinc fingers can bind to DNA with high specificity, and they can also interact with other proteins.
- This makes them essential for a wide range of cellular processes, including gene regulation, DNA repair, and protein folding.
Zinc finger motif: The zinc finger motif is a small protein structural motif that is characterized by the coordination of one or more zinc ions. It is found in many different proteins, including transcription factors, RNA binding proteins, and DNA repair proteins. Zinc fingers can bind to DNA with high specificity, and they can also interact with other proteins. This makes them essential for a wide range of cellular processes, including gene regulation, DNA repair, and protein folding.
- Zinc finger motif consists of an α helix and two short antiparallel β strands all held together by a zinc ion, coordinated between two conserved cysteine and two histidine side-chains. Some forms of zinc finger have four cysteine residues bound to the zinc ion. This motif can be repeated many times in a DNA binding protein, with each finger folding independently. It is amino acids in the α helix that interact with the major groove of the DNA duplex.
- Leucine zipper: The leucine zipper is another protein structural motif that is found in DNA binding proteins. It consists of two identical α helices that are held together by hydrophobic interactions between side-chains, notably those of leucine residues. The leucine zipper forms a short stretch of coiled-coil, which can then interact with another leucine zipper on another protein. This interaction can help to bring two proteins together, which can then regulate gene expression.
Protein Coiled Helices
Coiled coils are α-helices that twist around each other, stabilized by hydrophobic interactions. Found in myosin, α-keratin, DNA-binding proteins.
The coiled-coil structure is a very important secondary structure in proteins. It is found in a variety of proteins with different functions, and it is essential for the structure and function of these proteins.
Coiled Helices

Torsion Angles
Zinc Fingers